What is an aurora?
16 November 2017
An aurora is one of nature’s most spectacular visual phenomena—mysterious bright lights dancing across the night sky. So what causes auroras, why are they only visible at certain places and what gives them their beautiful colours?
Video: Watch this short video to understand more about auroras.
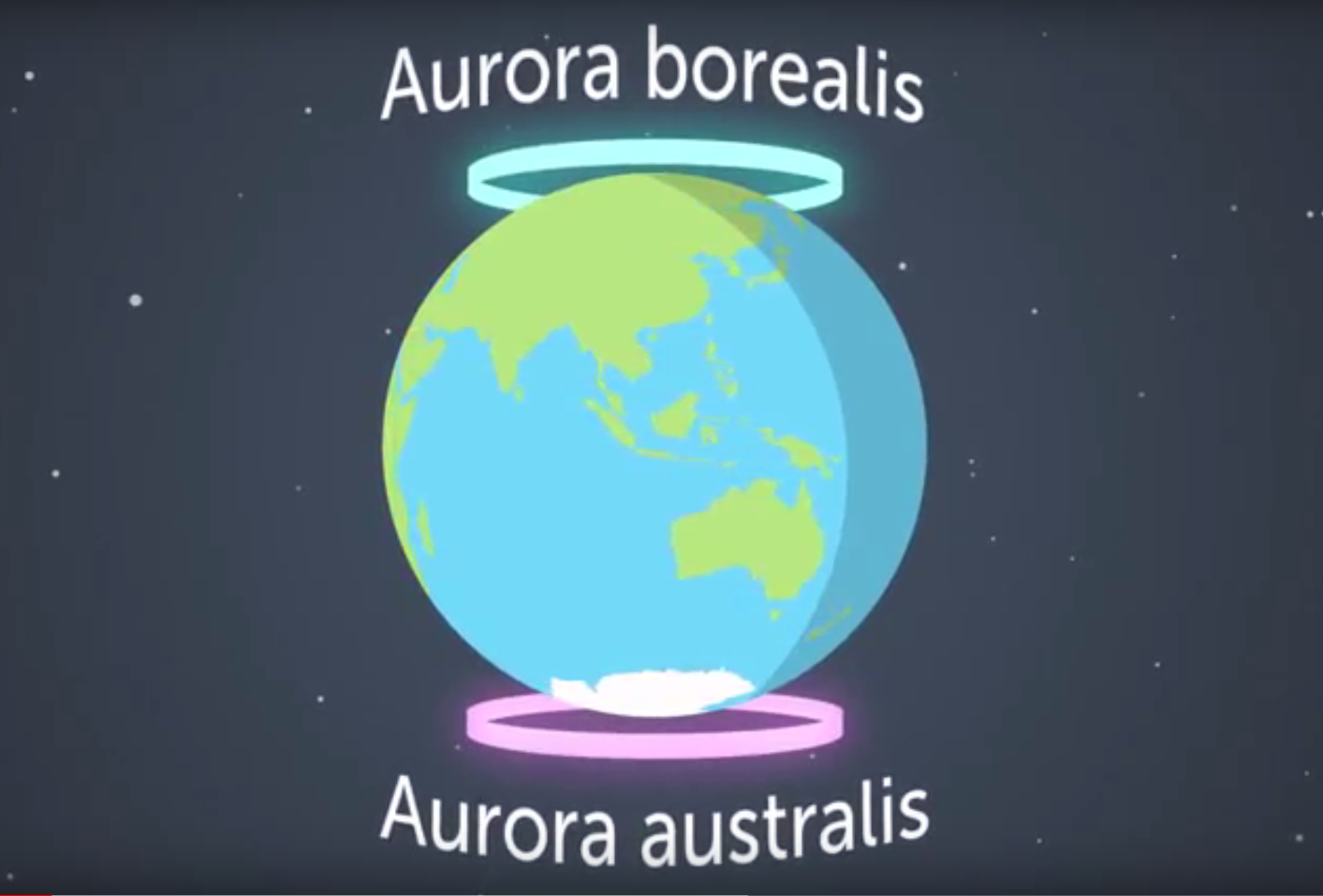
The brightest auroras are concentrated in rings, called the auroral ovals, around the north or south poles. The auroras in the northern hemisphere are called aurora borealis (or Northern Lights). In the southern hemisphere, they’re called the aurora australis (‘australis’ comes from the Latin word for ‘southern’) or Southern Lights.
Image: the auroral ovals
Born in a solar storm: where does an aurora begin?
Auroras are the result of events that begin on the Sun, millions of kilometres from Earth. The Sun is so hot that high-energy plasma (a gas of electrically charged particles) can escape from its gravitational field—this is called the solar wind. When the Sun is relatively inactive, the solar wind travels at about 300–400 km per second. When the Sun is more active, eruptions can occur that release dense, magnetised clouds of plasma into the solar wind. These plasma clouds travel away from the Sun (sometimes in the direction of Earth) at speeds of up to 2000 km per second—this is known as a solar storm.

Image: Solar eruptions carry magnetised plasma into space. Credit: NASA
Magnetic interaction: how does the light happen?
The solar wind has enough energy that it could strip away the Earth’s atmosphere over time. Luckily, the Earth has a magnetic field due to the hot, electrically charged, molten rock swirling around in its outer core. The interaction of the solar wind with this magnetic field creates a protective cavity called the magnetosphere, which deflects most of the particles from the solar wind.
When a solar storm reaches Earth, the fast-flowing plasma temporarily deforms our magnetosphere, causing a build-up of energy on the night side of the Earth. When conditions are right, this energy is released, accelerating charged particles so they spiral around the magnetic field lines toward the north and south poles. When these particles reach our upper atmosphere, they collide with neutral atoms of oxygen and nitrogen. During these collisions, the charged particles transfer energy to the atoms, ‘exciting’ electrons in those atoms to higher energy levels. When the electrons return to their original energy level, they release this energy in the form of light.

Image: The aurora australis at Aireys Inlet, Victoria on 28 March 2017. Credit: Lachlan Manley Photography
Atomic palette: what makes the beautiful colours?
Light is released by the atoms at various wavelengths—and the wavelengths determine its colour. The colour emitted depends on how energetic the collisions are, where they occur in the atmosphere and which atoms and molecules are involved. Oxygen releases greenish-yellow or red light, while nitrogen releases dark red or blue light. These colours can mix—so purple, pink and white light can also be seen...if you are lucky enough to catch the elusive aurora!



Comment. Tell us what you think of this article.
Share. Tell others.